Multiple System Atrophy
Multiple system atrophy (MSA) is a rare neurodegenerative disorder affects up to 17,000 individuals in the U.S. and an estimated 23,000 in the EU. New cases of MSA in the U.S. are estimated at 1,900 per year. No specific genetic defect or other cause has been discovered leading to this disorder, and the cause is likely a combination of genetic tendencies and environmental factors. Clinical features of the disease typically appear between 55 and 60 years of age. MSA is a rapidly progressive condition, with a mean survival from symptom onset of six to 10 years.
Typical signs of the MSA include blood pressure variability, urinary incontinence, parkinsonism, poor balance, sleep dysfunction, incoordination of arms and legs and spasticity. The two forms of MSA are MSA-P and MSA-C. Patients with MSA-P predominantly show symptoms similar to Parkinson’s such as tremors, muscle rigidity and slowness of voluntary movements. Those patients with MSA-C show signs of cerebellar dysfunction that results in a lack of control of involuntary functions and muscle movements in the limbs, or what is referred to as Ataxia. MSA-C affects 60%-80% of individuals with MSA.
Clinical Trials
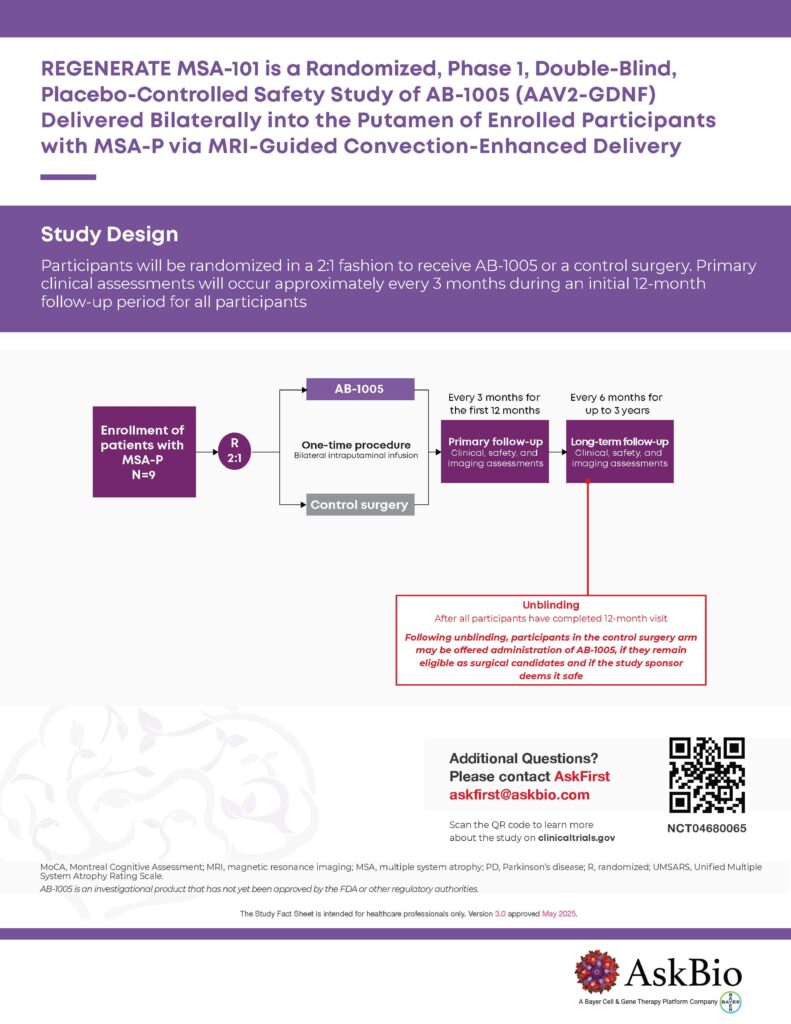
We are now enrolling patients with the parkinsonian subtype of MSA (MSA-P) to assess the safety, tolerability and preliminary efficacy of AB-1005 gene therapy for this rapidly progressing condition. MSA is a rare neurodegenerative disorder. At AskBio, we are working to advance new treatments as quickly and safely as possible.
Understanding MSA
When an individual has MSA, it is the result of a build-up of a protein that supports the nerve cells in your brain, largely responsible for controlling both voluntary and involuntary movement and function. While similar to Parkinson’s, in that MSA will affect the production of dopamine, a chemical that carries messages around your brain to your nerve cells that control body movements, MSA is characterized by degeneration of multiple neurological systems due to the impact on neurons that support healthy production of nerve growth factors. All with MSA will ultimately suffer from complete automimic failure, the systems in the body that control functions such as blood pressure, going to the bathroom, digestion, sweating, breathing – all those functions we do without having to think about. It is a rapidly progressive disease that causes deterioration of three core parts of the brain:
- The structures in the brain that links controls for movement learning, memory, and emotional processing, known as the basal ganglia.
- The brains lower region, the brainstem, that controls breathing, heart rate, blood pressure and other involuntary processes.
- In the back of the brain, the cerebellum that is responsible for coordinating movement and balance.
It is often challenging to distinguish between Parkinson’s Disease and MSA, especially in early stages of MSA, but MSA symptoms will progress more rapidly and severely than Parkinson’s. One distinguishable symptom that is common with the majority of Parkinson’s patients is tremors at rest but, is not common with most MSA patients. In addition, MSA patients will present facial muscle decline, severe sleep disorders, jerky muscle movements, postural abnormalities, speech and swallowing difficulties and involuntary and uncontrollable emotional responses.
Visit the National Institutes of Health Medline Plus website to learn more about MSA.
Gene therapy for MSA
By enhancing levels of glial cell line-derived neurotrophic factor (GDNF), a naturally occurring growth factor in the brain, AB-1005 gene therapy is intended to promote the survival and functioning of vulnerable dopamine-producing brain cells that are “sick but not dead” in MSA-P. Taking advantage of the brain’s own cellular production, the therapeutic is designed to encourage natural growth of dopamine cells with a goal to restore continuous production of GDNF protein, necessary for the recipient nerve cells to trigger healthy body movement and various functions. The proposed gene therapy, AB-1005, may provide an advantage over intermittent protein infusions of synthetic GDNF, where brain levels may become lower than effective levels between infusions.
Direct brain delivery of the AB-1005 therapeutic agent is a specialized operative procedure that uses customized real-time MRI to allow the surgeon to see a clear visual path for accurate distribution of the therapeutic payload. The procedure is tailored to each individual’s precise anatomical requirements.