Creating a paradigm shift in raw materials
Synthetic biology based on natural genomic knowledge has wide-ranging potential to advance how we bring more effective and safer therapeutics to patients. It allows us to treat more patients by speeding production and lowering cost.
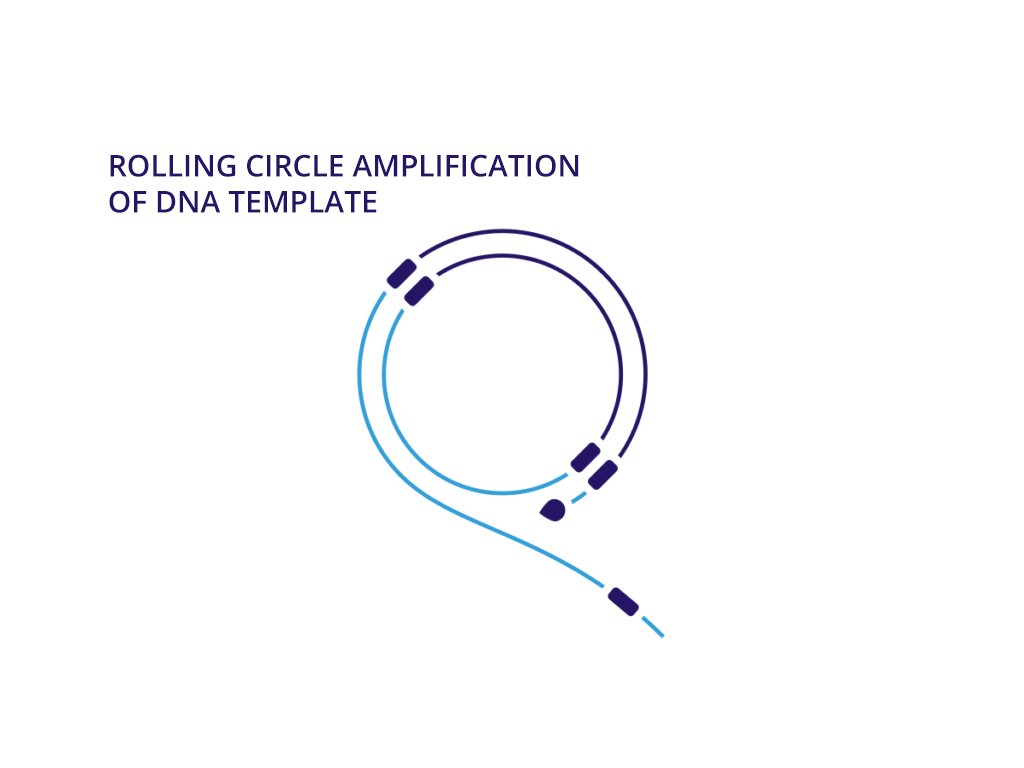
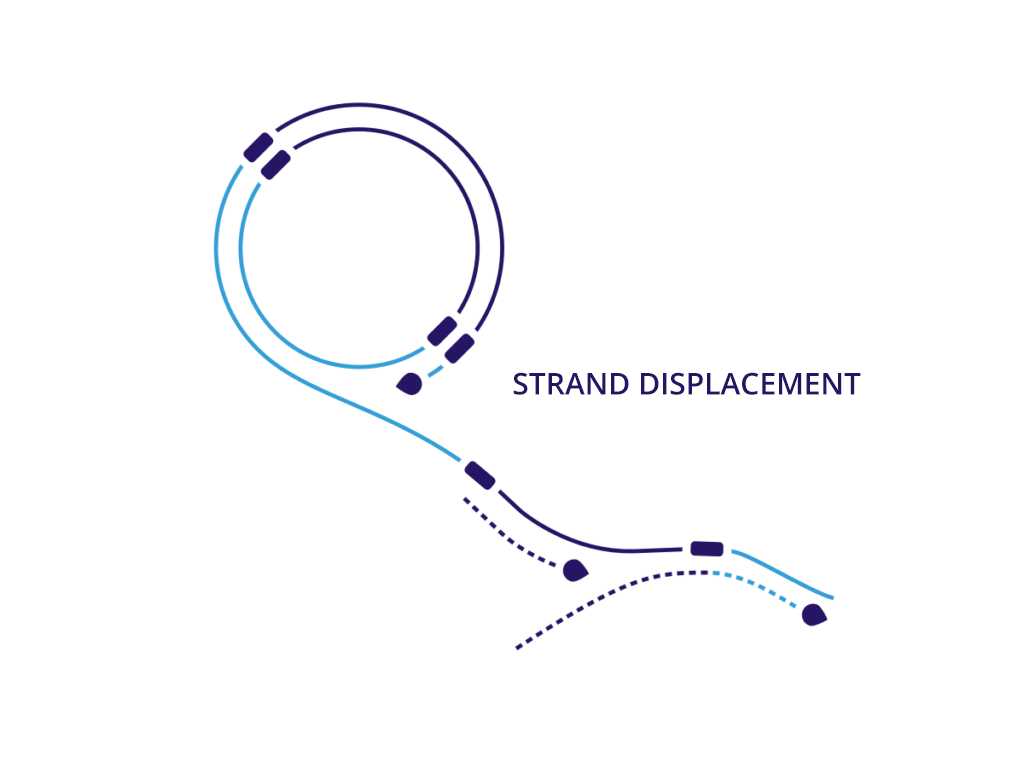
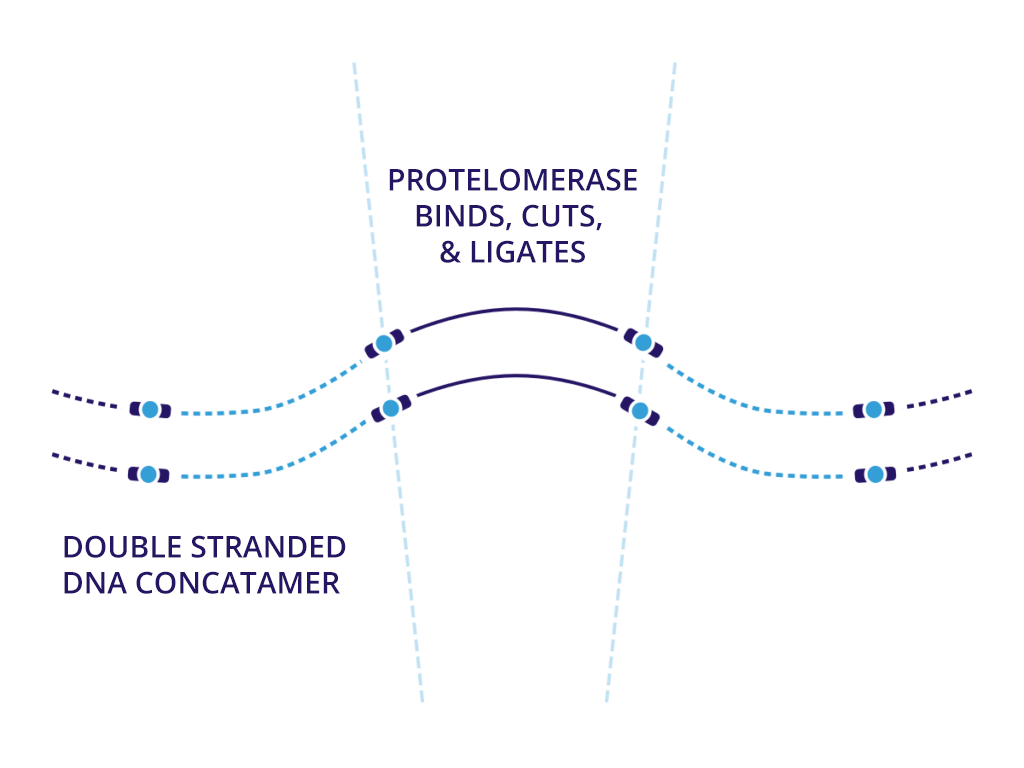
Enzymatic DNA does not require larger fermentation volumes of plasmids. This means we can generate DNA with no bacterial impact that can be produced in higher volumes in significantly less time than plasmid DNA. More importantly, we can assemble safer DNA sequences at a larger scale with the same therapeutic effect of natural synthesized DNA.
Working collaboratively with TAAV Biomanufacturing Solutions, AskBio is advancing neDNA™* into clinical applications with extensive development and manufacturing capabilities.
*Technology for making neDNA™ is licensed from Touchlight IP Ltd
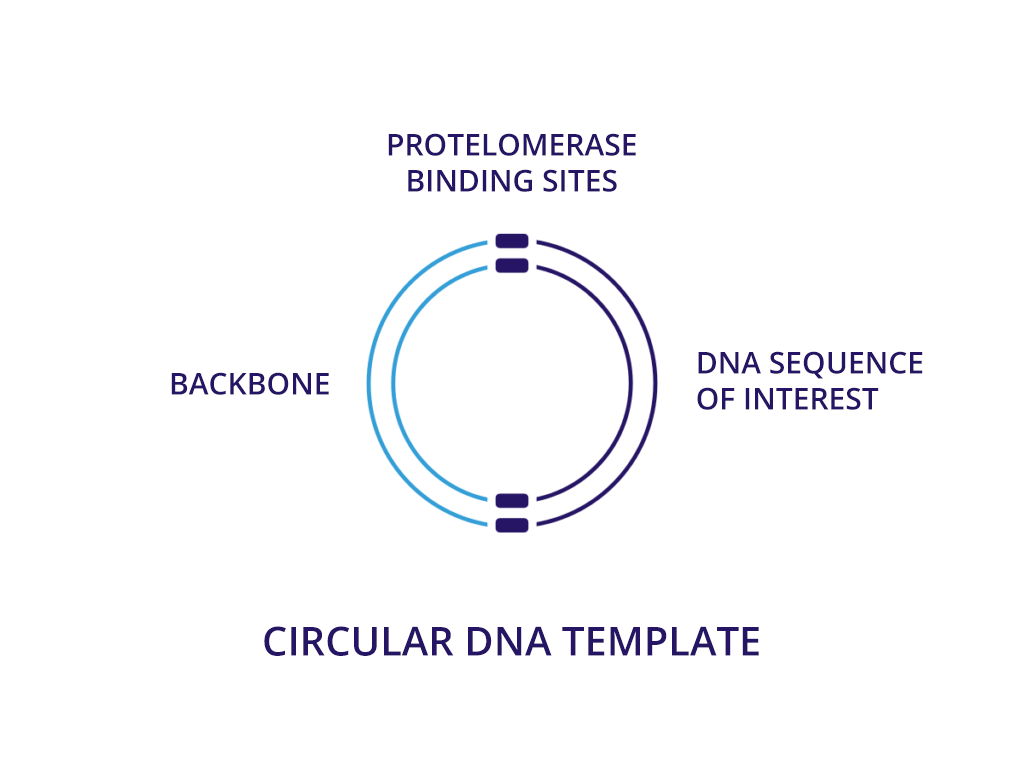
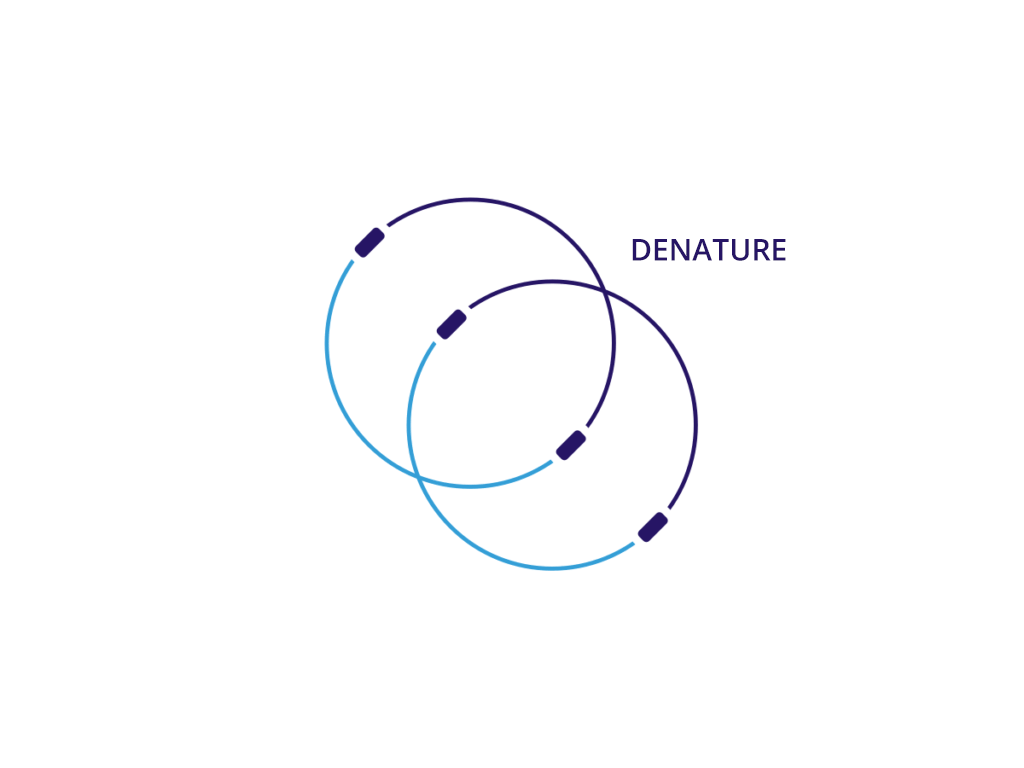
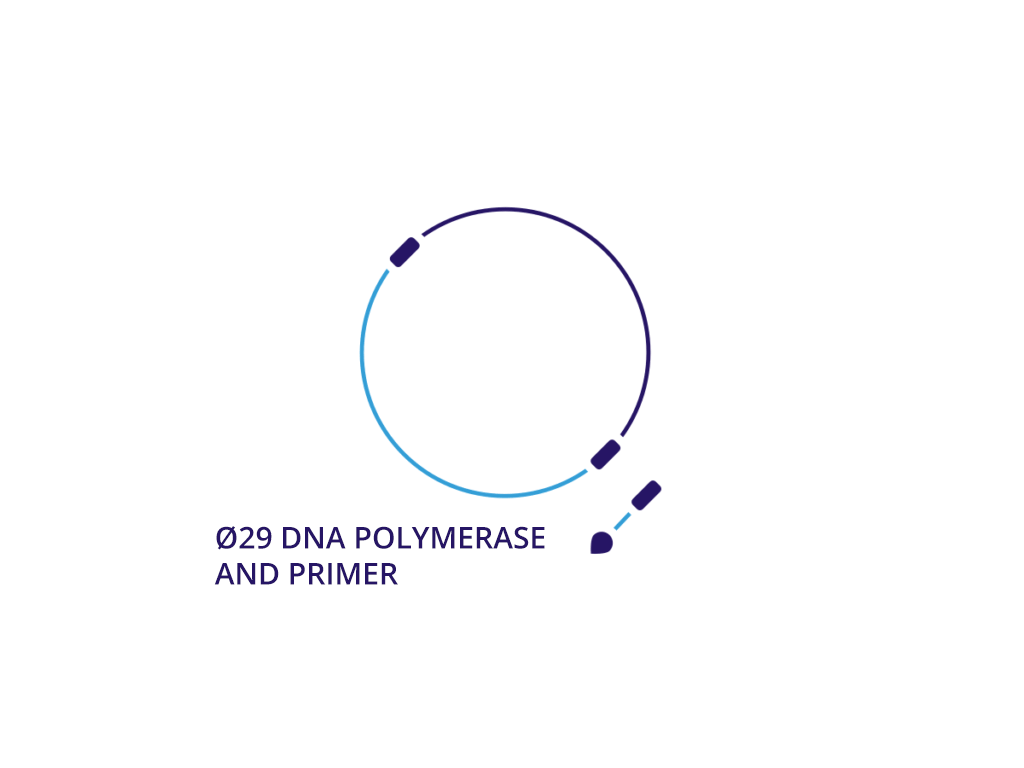
We believe that neDNA™ will offer an improved safety profile versus plasmid DNA for the manufacture of recombinant adeno-associated virus (rAAV) gene therapy vectors. The TAAV manufacturing process consistently produces large amounts of neDNA using technology licensed from Touchlight IP Ltd.* in reduced times, which can be measured in days versus the months that are typical when producing plasmid DNA. neDNA features a novel, minimal linear double-stranded DNA vector. It’s closed ends improve expression characteristics. It is capable of long and difficult sequences and eliminates bacterial sequences.