Better AAV therapeutics
The promise of AAV gene therapy comes with an obligation to solve the complicated issue of supplying viral-based gene therapies on a global scale. AAV manufacturing is highly complex and historically inefficient with a cost of goods that pushes the price of therapeutics into the millions of dollars. We recognized these issues more than a decade ago and focused our efforts on overcoming these obstacles. The result was Pro10™ technology and the foresight to create world-class GMP manufacturing facilities.
We’ve set the industry standard for scalable, reliable and efficient manufacturing to help drive down production cost and expand investigation of therapies for patients with unmet needs.


Clinical & commercial AAV manufacturing
Our independently operated Viralgen subsidiary is a cGMP manufacturing facility. Viralgen Vector Core uses our Pro10™ cell line technology to manufacture AAV in a state-of-the-art facility for use in human gene therapy clinical trials. Viralgen offers what we believe is the most flexible, scalable AAV manufacturing system in the industry.
We continue to invest in innovative AAV manufacturing at Viralgen, our focused CDMO organization in San Sebastian, Spain, as well as our headquarters in Research Triangle Park, NC.

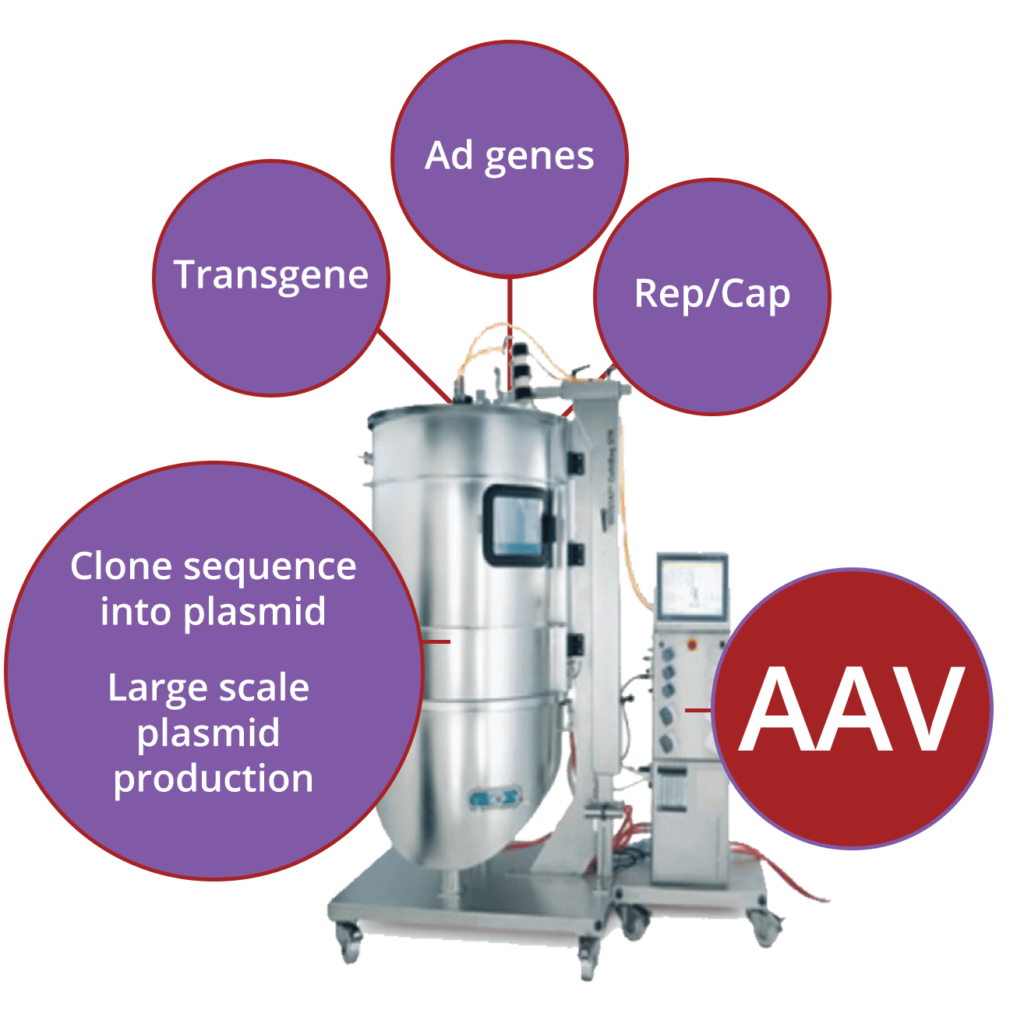

neDNA™ manufacturing
AskBio and its subsidiary, TAAV, supply the rapidly expanding AAV market with proprietary neDNA™*. An alternative to traditional transfection DNA substrate and a critical starting material, we are setting new standards for synthetic DNA best practices. Our high-yield manufacturing process lowers cost, expedites production and increases safety without prokaryotic DNA sequences.
*Technology for making neDNA™ is licensed from Touchlight IP Ltd
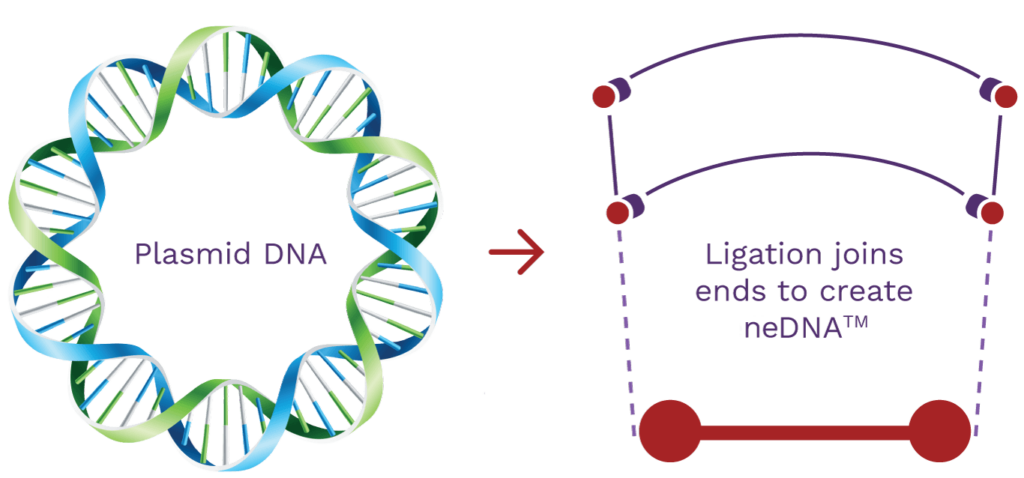
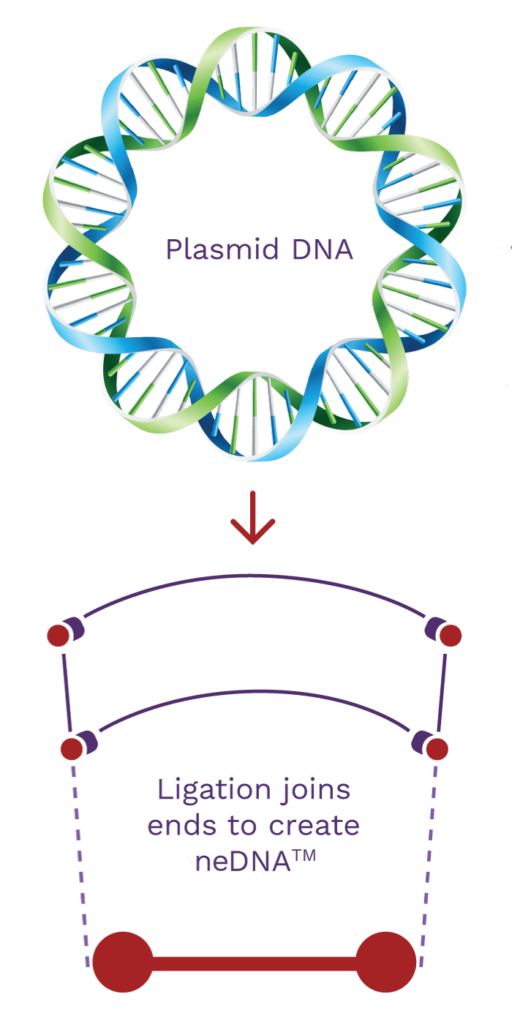
Synthetic neDNA™
A synthetic bench-top process alternative to plasmid DNA.